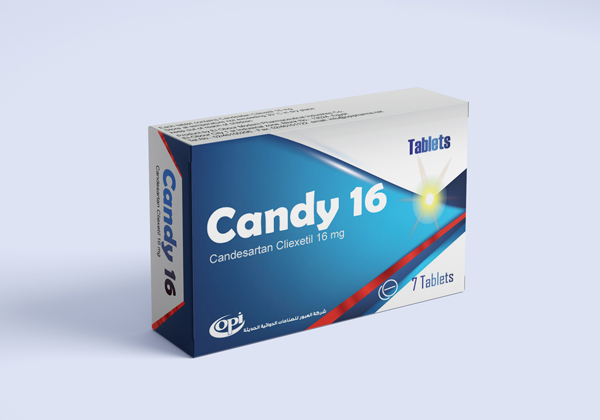
Candy 16 mg
These preparation are not used during pregnancy as they may cause fetal injury or fetal death
Name of the medicinal product
CANDY 8MG TABLETS
CANDY 16 MG TABLETS
Qualitative and quantitative composition
Each tablet contains 8 mg Candesartan Cilexetil .
Each tablet contains 16 mg Candesartan Cilexetil.
Excipient with known effect
-
- 8 mg: Each tablet contains (lactose monohydrate 70mg )16 mg: Each tablet contains (lactose monohydrate 164mg )
- Pharmaceutical form
Tablet.
Candy 8mg :(White round biconvex tablet scored from one side )
Candy 16 mg : (White , round, biconvex tablet scored from one side)
- Clinical particulars
4.1 Therapeutic indications
- Treatment of essential hypertension in adults.
- Treatment of adult patients with heart failure and impaired left ventricular systolic function (left ventricular ejection fraction ≤ 40%) when Angiotensin Converting Enzyme (ACE)-inhibitors are not tolerated or as add-on therapy to ACE-inhibitors in patients with symptomatic heart failure, despite optimal therapy, when mineralocorticoid receptor antagonists are not tolerated (see sections 4.2, 4.4, 4.5 and 5.1).
- Treatment of hypertension in children and adolescents aged 6 to <18 years.
4.2 Posology and method of administration
- 8 mg: Each tablet contains (lactose monohydrate 70mg )16 mg: Each tablet contains (lactose monohydrate 164mg )
Posology in hypertension
Elderly population
Renal impairment
Hepatic impairment
Black patients